Real-World Evidence (RWE) in healthcare is emerging as a cornerstone of medical innovation. It relies on the analysis of real-world data – such as the SNDS (French National Health Data System), registries, or medical records – outside of traditional clinical trials. These data help better understand the use and effectiveness of health products in real-life conditions, across large and representative populations.
What is healthcare RWE?
Healthcare RWE refers to all evidence generated from real-world data. Unlike clinical trials, it reflects patients’ everyday lives. It draws on various sources: medico-administrative databases, registries, electronic records, etc. These data are analyzed to assess treatments, care pathways, and clinical outcomes.
Why is healthcare RWE strategic?
Healthcare RWE has become a key driver for:
– Pharmaceutical and medical device companies
– Payers and health authorities
– Researchers and academic teams
– Patients themselves
It helps guide decision-making, optimize therapeutic strategies, and strengthen trust in innovations.
How does our RCTs team excel in this field?
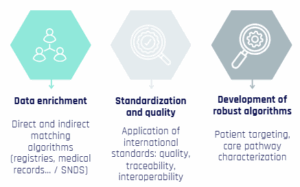
External validation and methodological excellence: Our RCTs team relies on recognized expertise in pharmacoepidemiology. We validate model accuracy, develop predictive tools, and use robust methods such as Cox models, propensity scores, or external comparators.
Internal data linkage and technical mastery: We perform indirect linkages between internal data, following international quality standards. Our selection, exposure, and analysis algorithms ensure scientific rigor and optimal reliability.
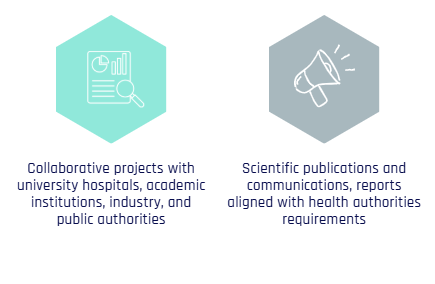
Collaborations and impact: Our collaborative projects with university hospitals, registries, academic and industrial partners, as well as with the HDH (Health Data Hub), lead to scientific publications, conference presentations, and reports valued by authorities.
Want to learn more?
Technical expertise
Achievements and impact